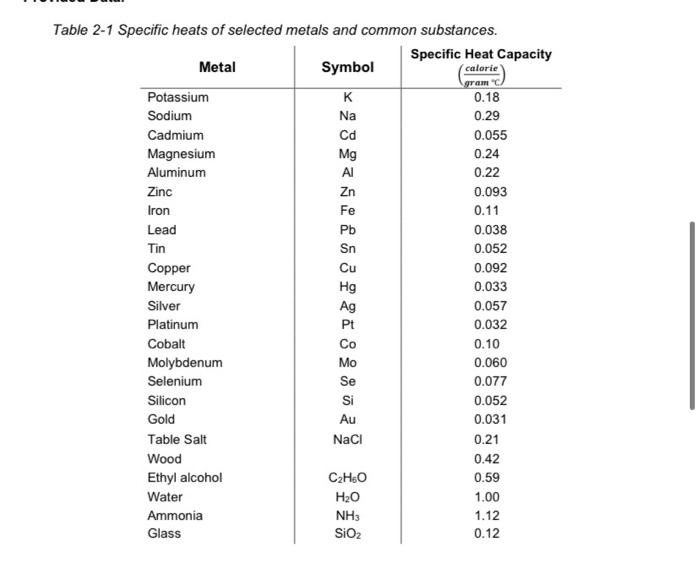
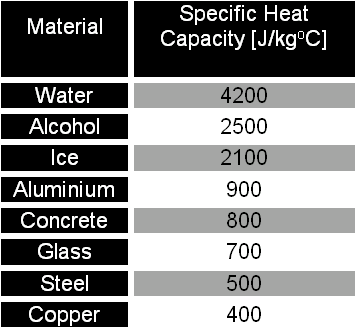
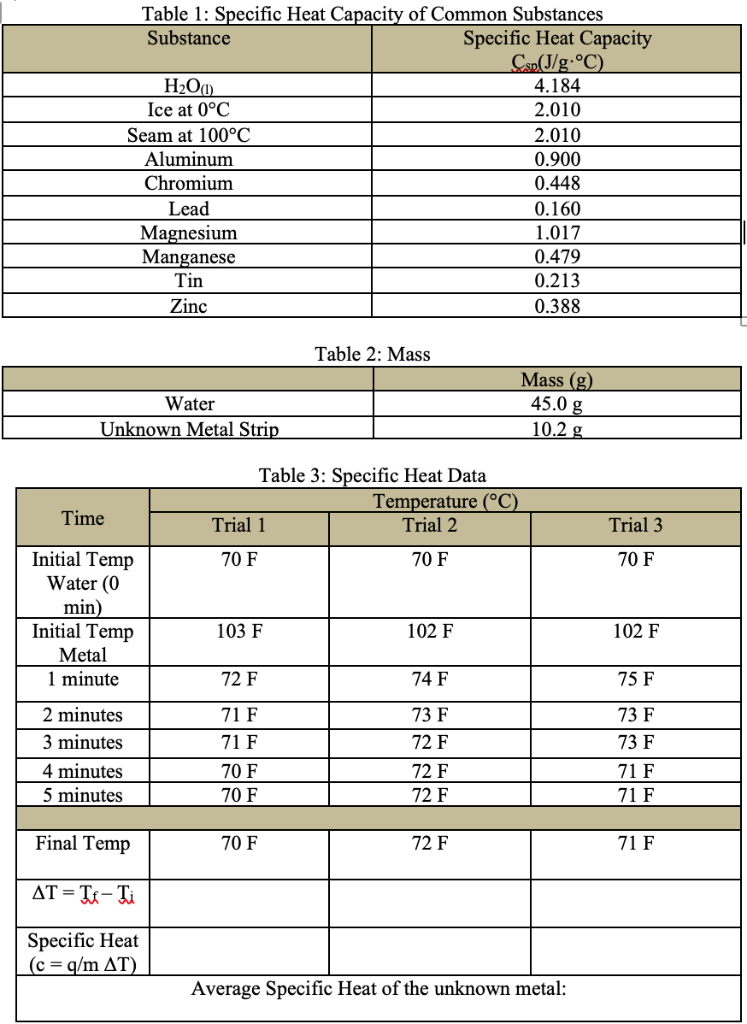
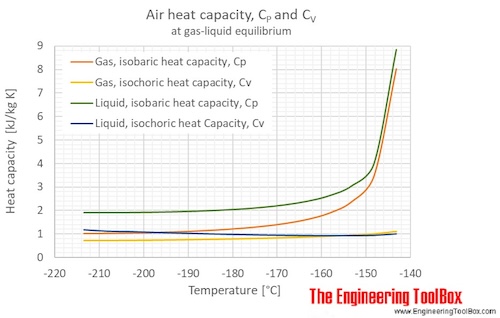
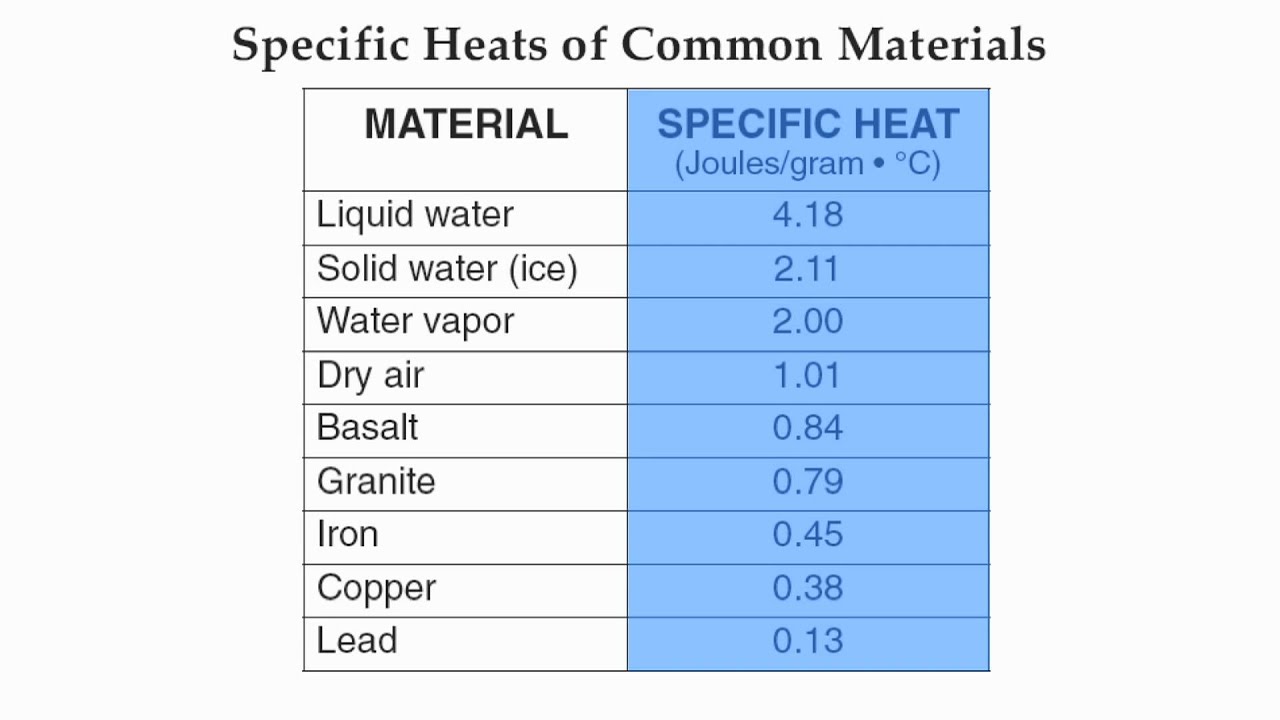
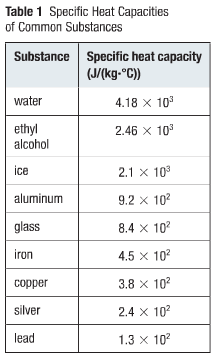
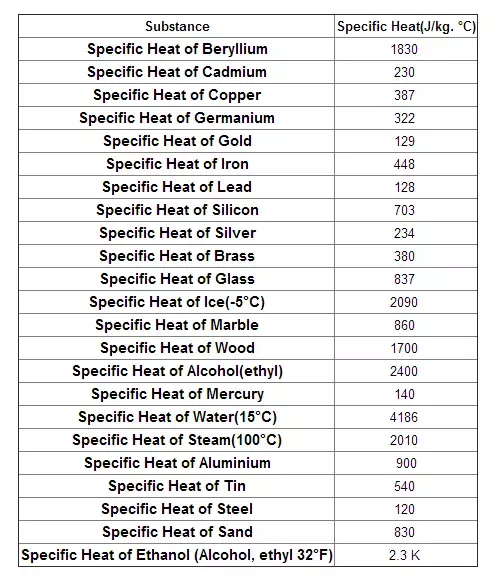
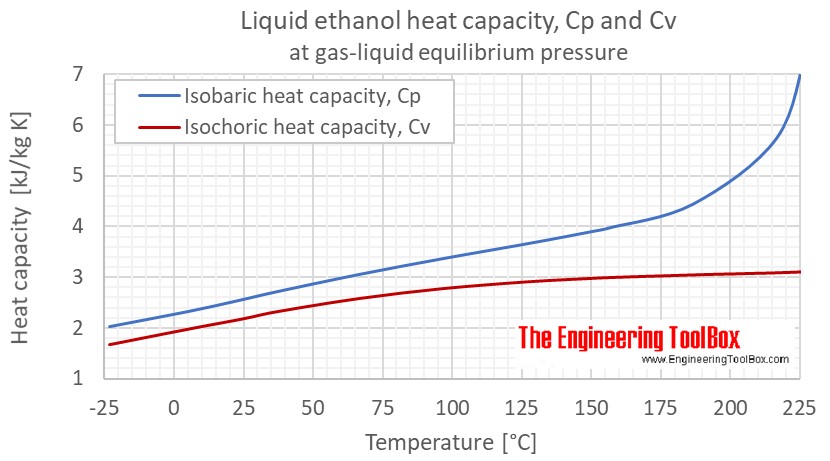
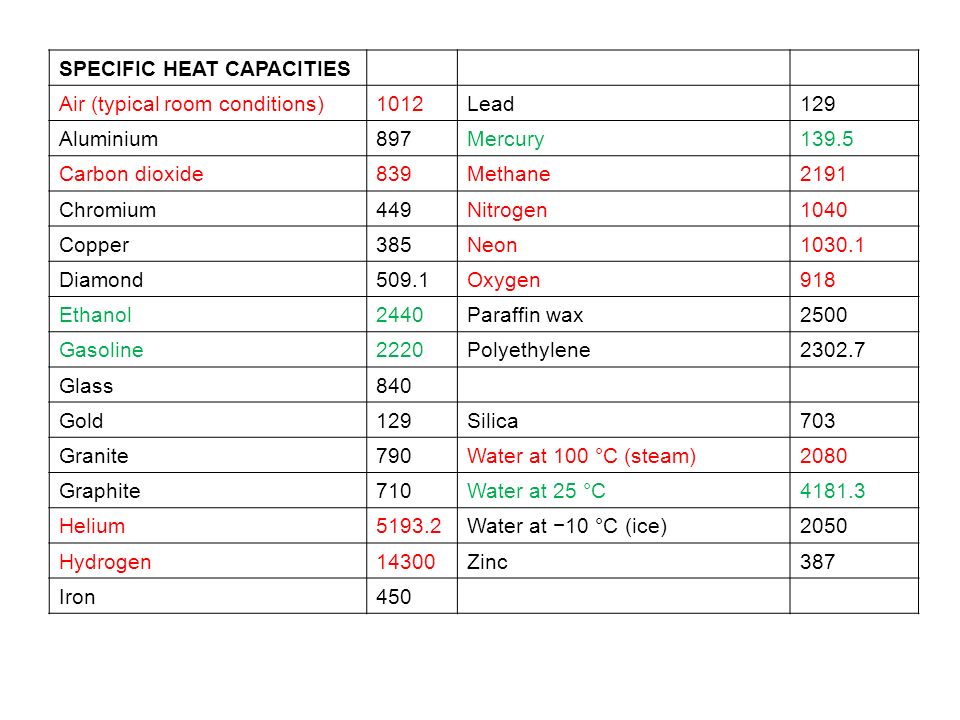
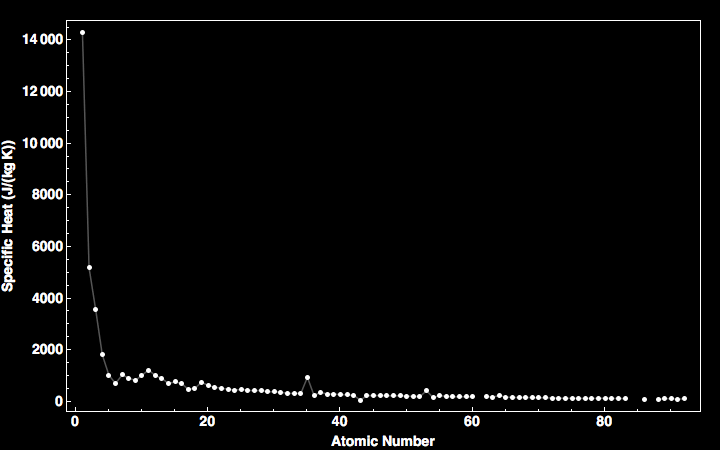
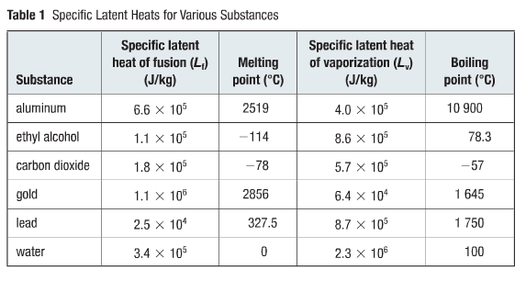
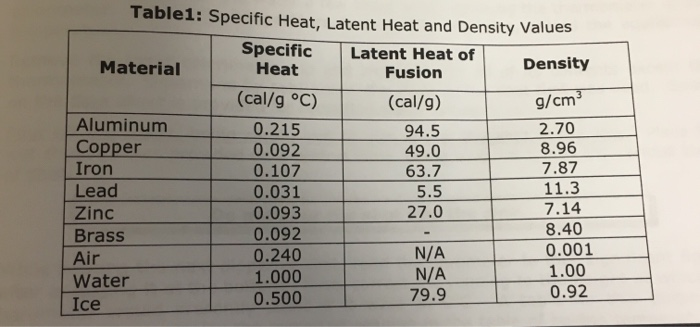
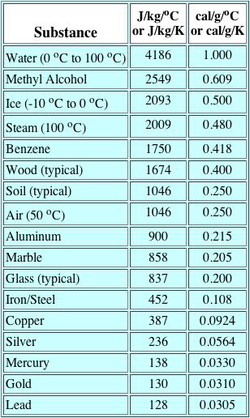
Specific Heat Capacity Chart
When heat capacity is measured per mole of a substance instead of measuring it per gram it is the molar heat capacity.
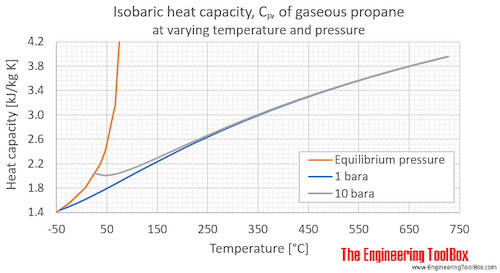
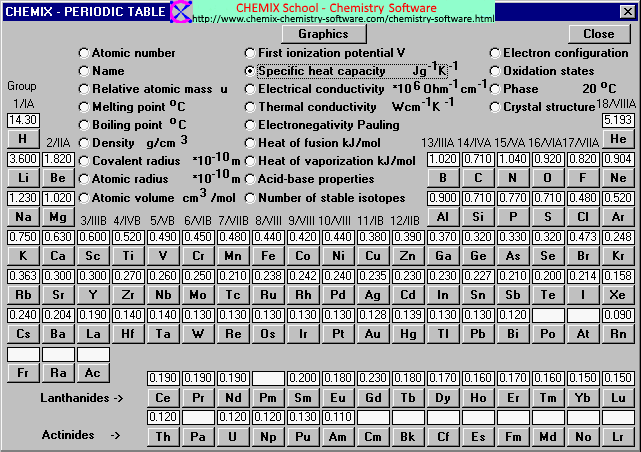
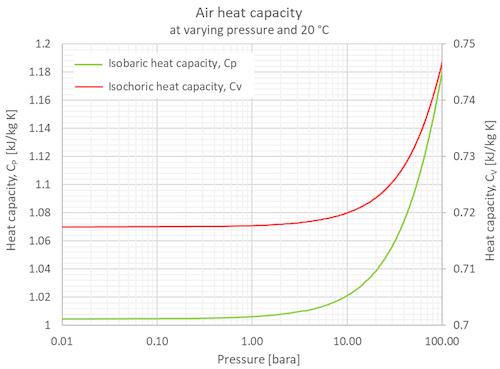
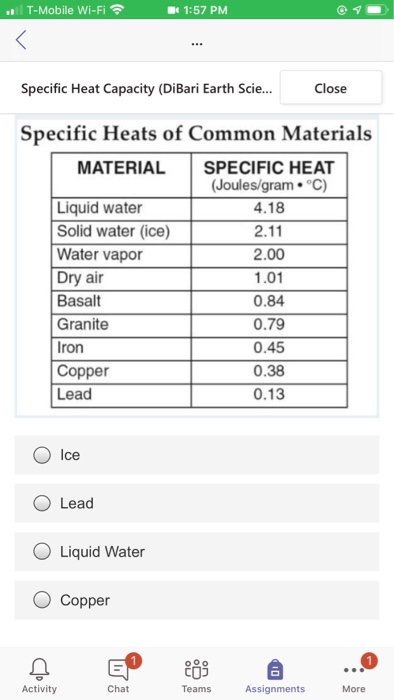
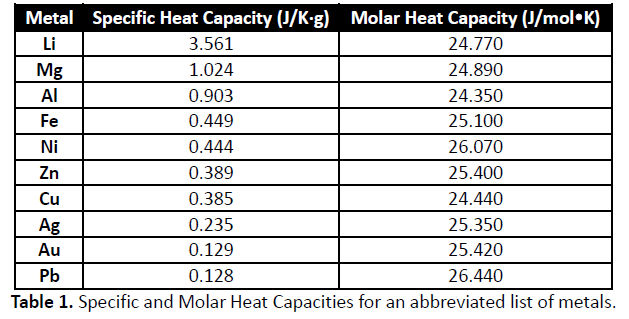
Specific heat capacity chart. The intensive properties c v and c p are defined for pure simple compressible substances as partial derivatives of the internal energy u t v and enthalpy h t p respectively. 1 btu lb f 4186 8 j kg k 1 btu lb f 4 1868 j g c. This specific heat chart table gives the specific heat of all the elements of periodic table in j kg k.
The point to be noted here is that the. The relationship between heat and temperature change is usually expressed in the form shown below where c is the specific heat. Specific heat or specific heat capacity is a property related to internal energy that is very important in thermodynamics.
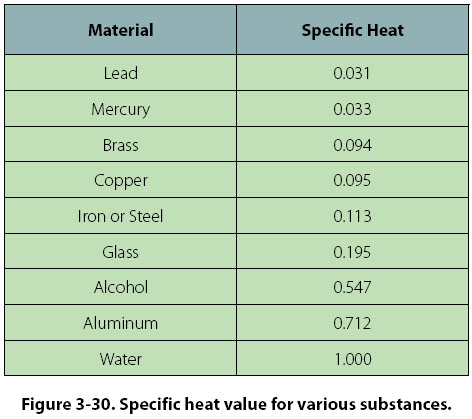
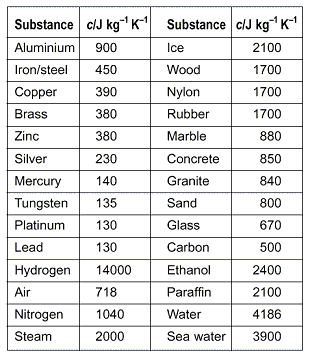
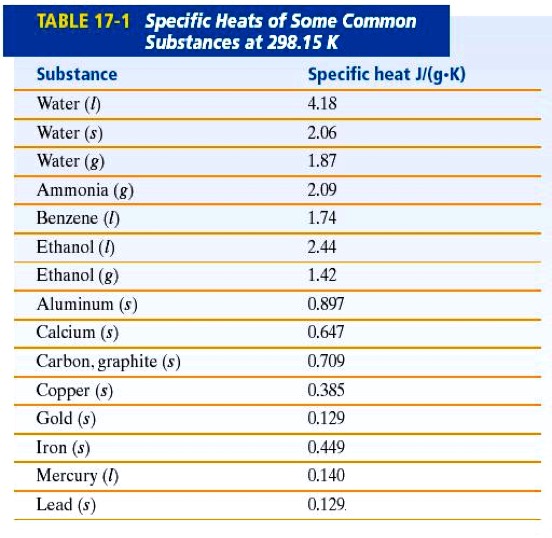
The following table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and engineering materials and when applicable the molar heat capacity. This table gives the specific heat capacity cp in j g k and the molar heat capacity cp in j mol k at a temperature of 25 c and a pressure of 100 kpa 1 bar or 0 987 standard atmospheres for all the elements for which reliable data are available. Generally the most constant parameter is notably the volumetric heat capacity at least for solids which is notably around the value of 3 megajoule per cubic meter and kelvin.
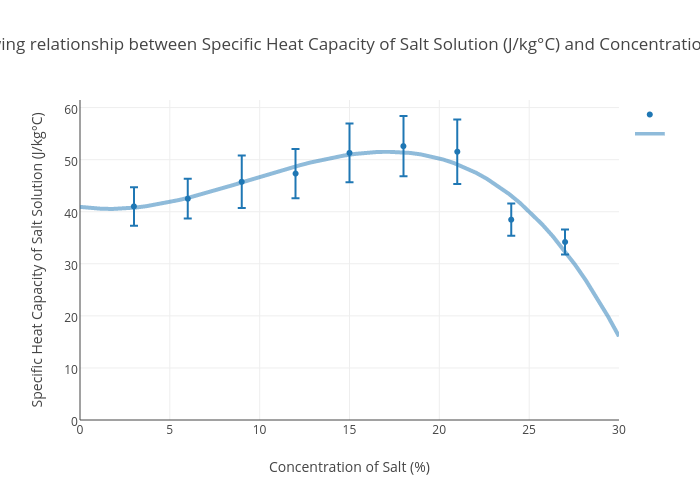
This value is always larger than specific heat capacity. Periodic table of elements with specific heat trends in the below periodic table you can see the trend of specific heat. Click on element atomic number element symbol element name and element specific heat headers to sort.