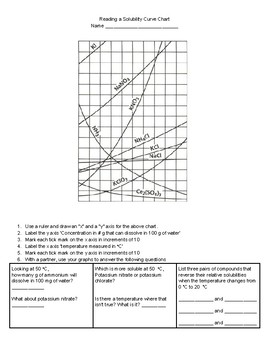
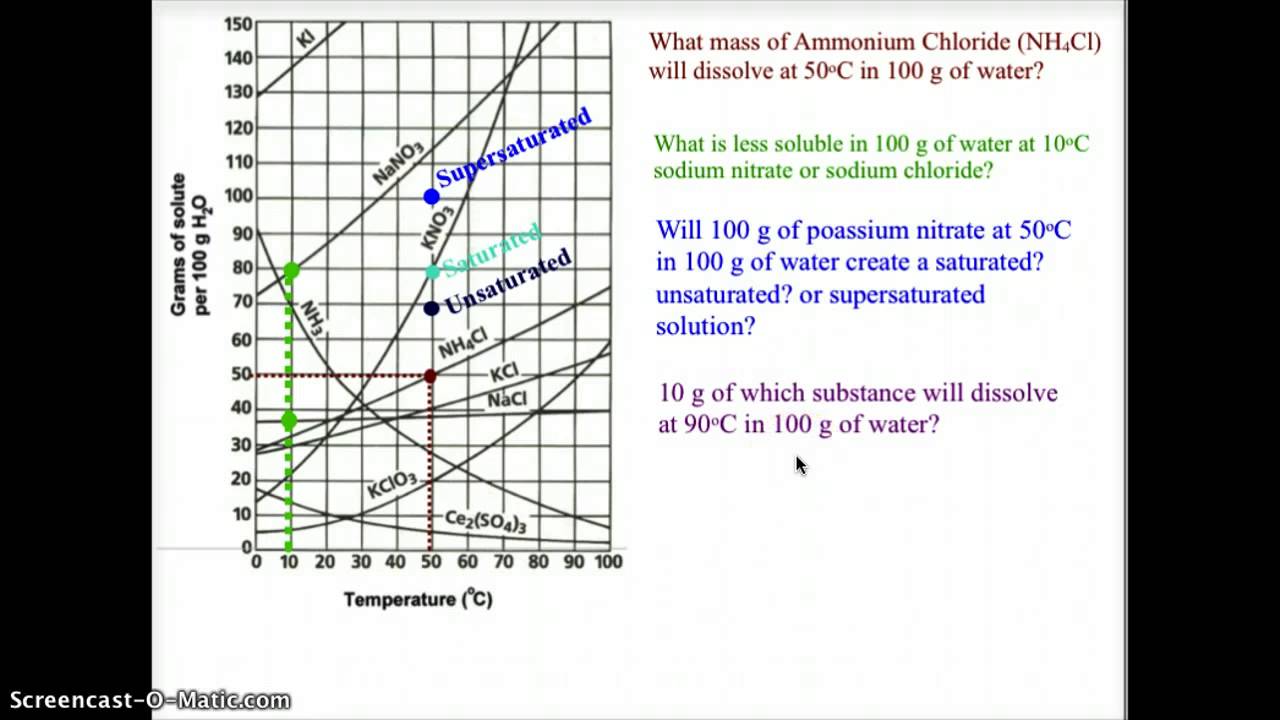
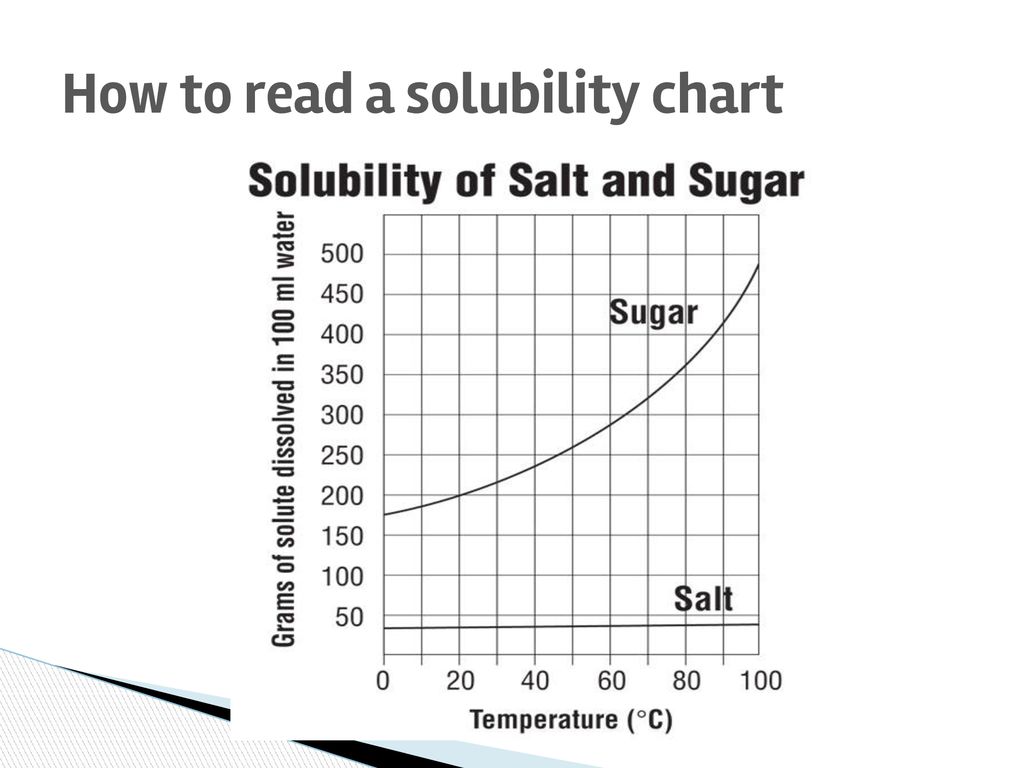
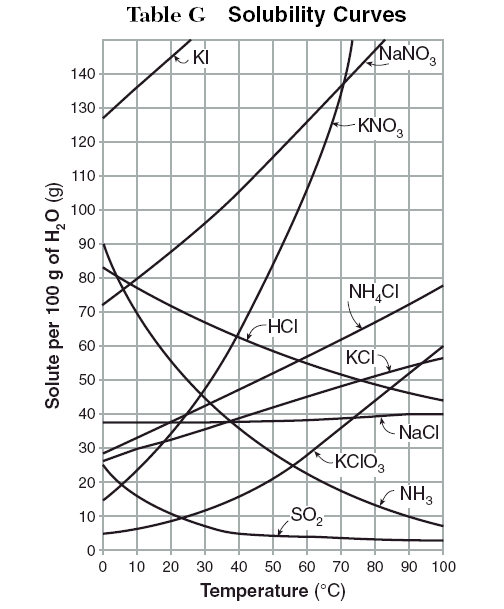
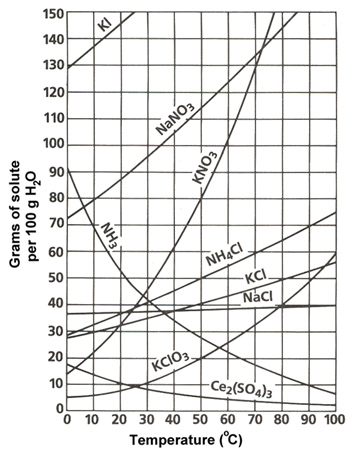
How To Read A Solubility Chart

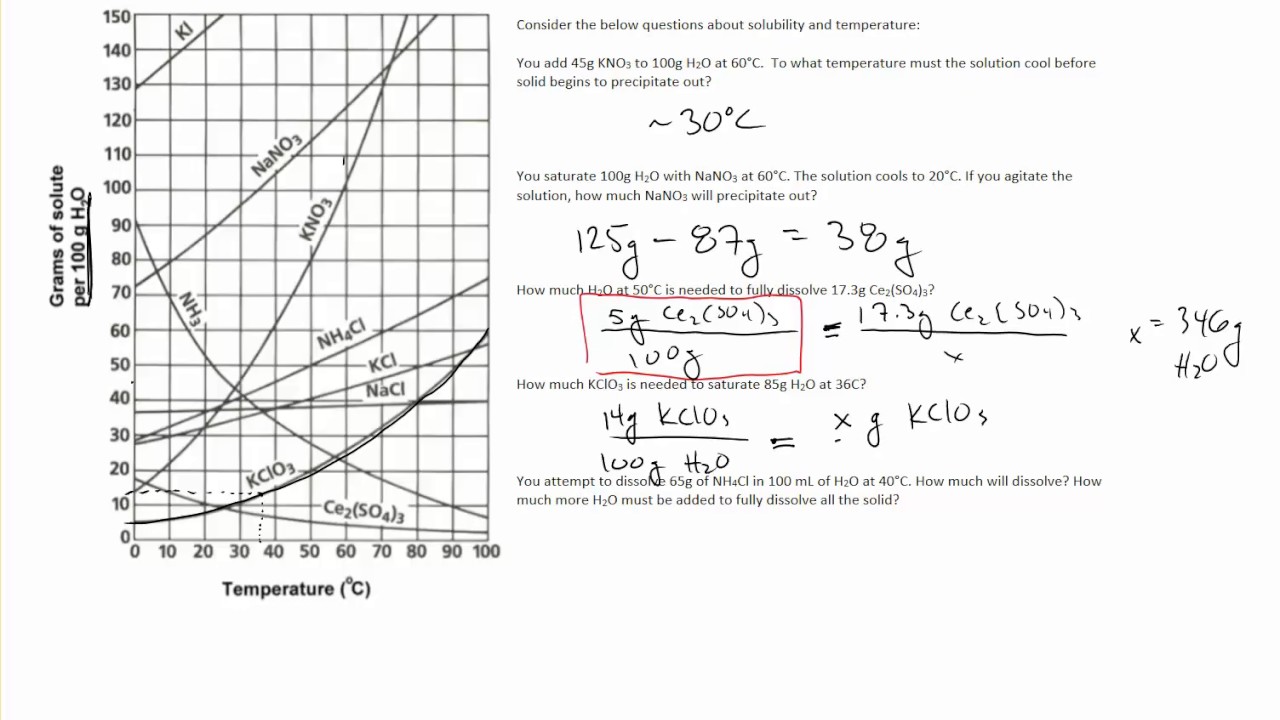
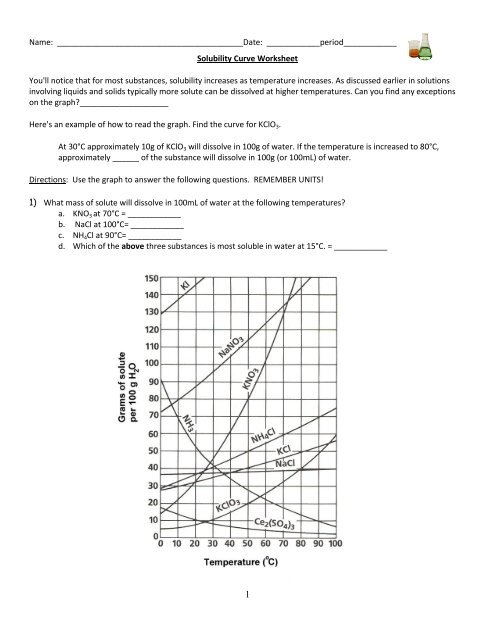
Reading solubility curves calculating solubility use the graph s mass and temperature to set up a proportion to estimate the solubility of a second mass or temperature.
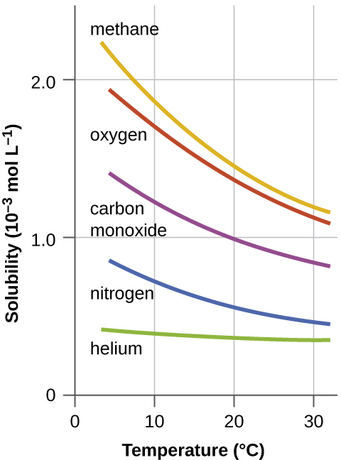
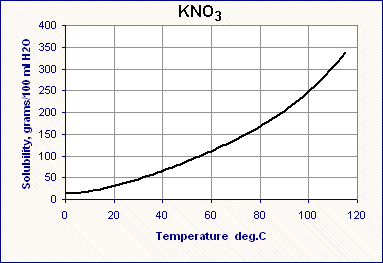
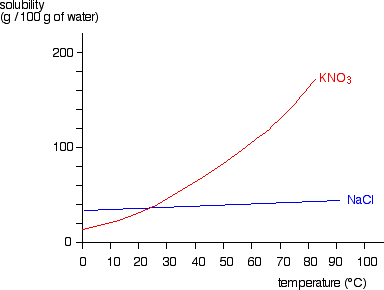
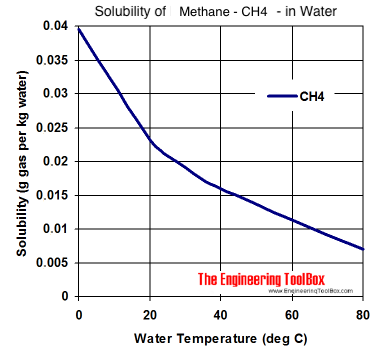
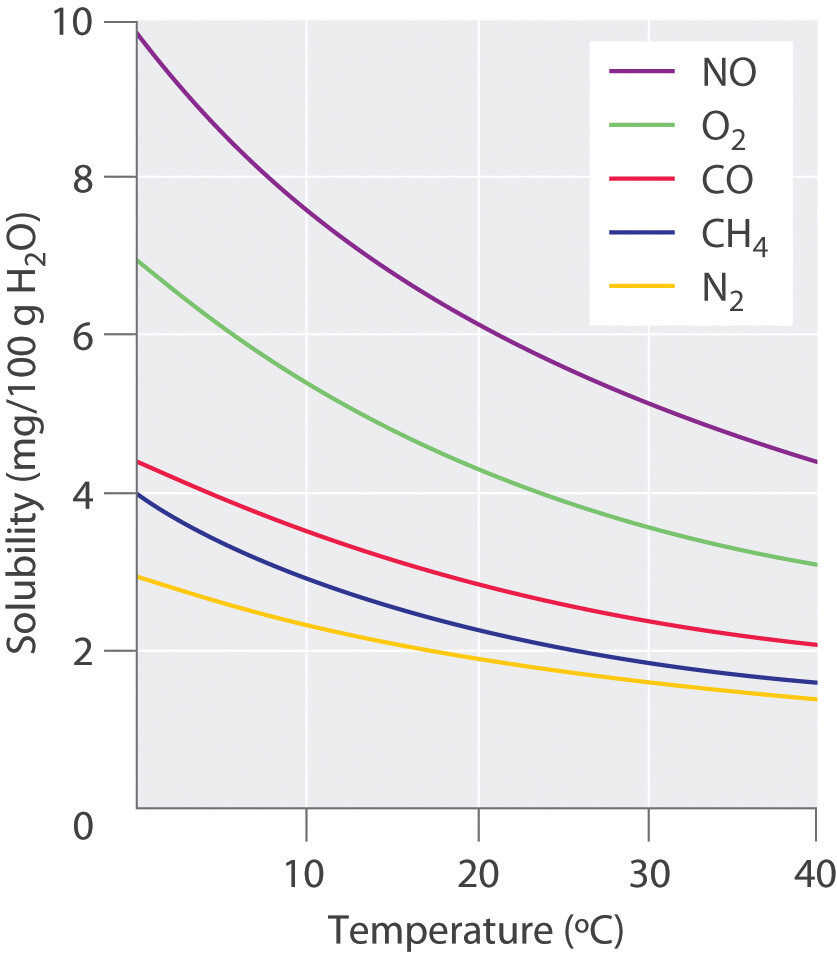
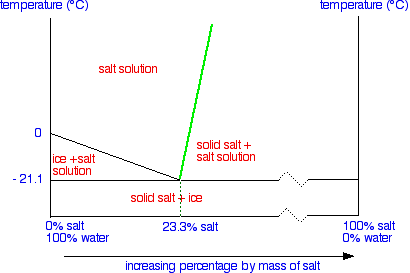
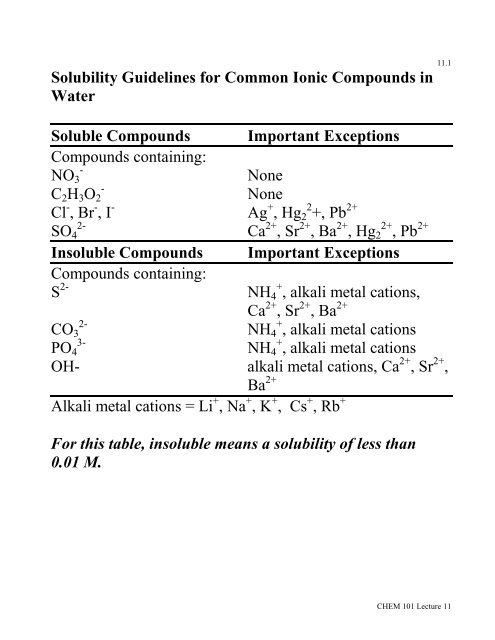
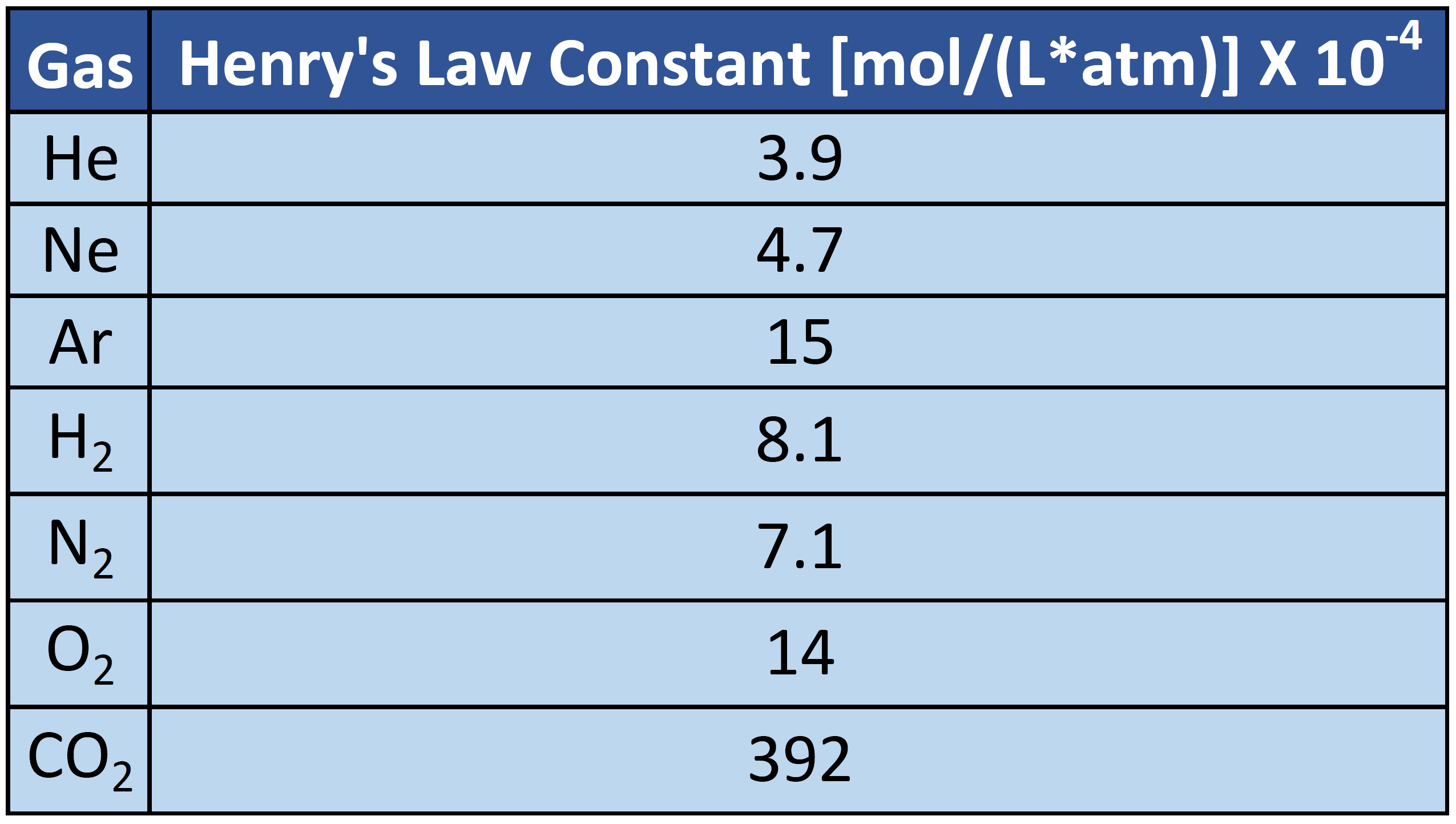
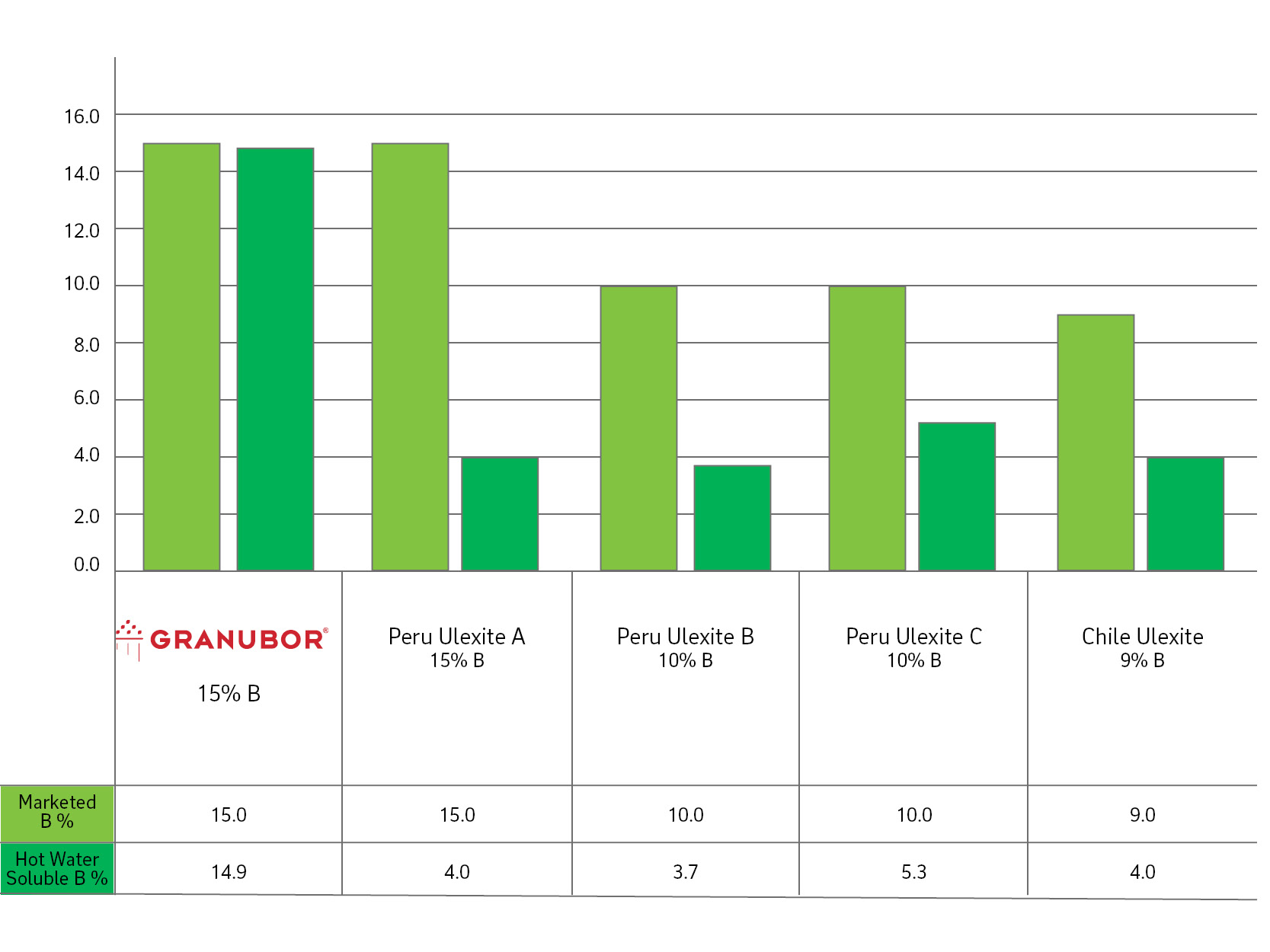
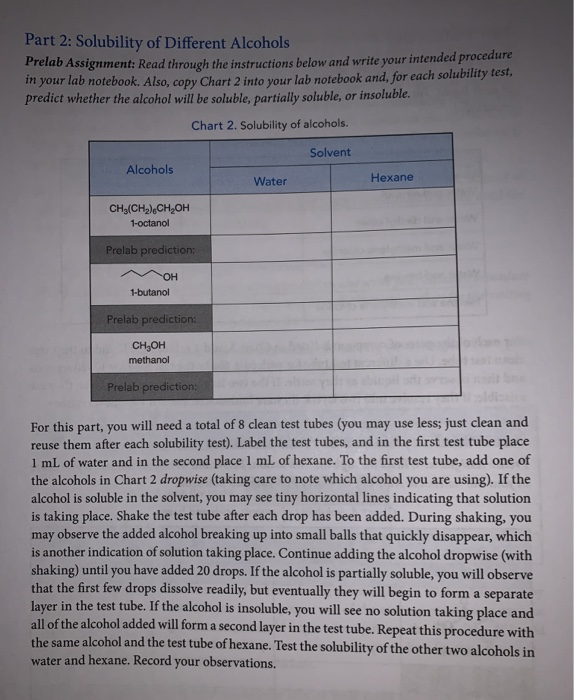
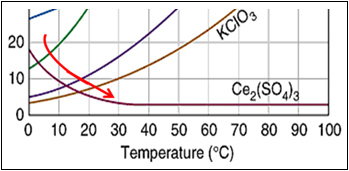
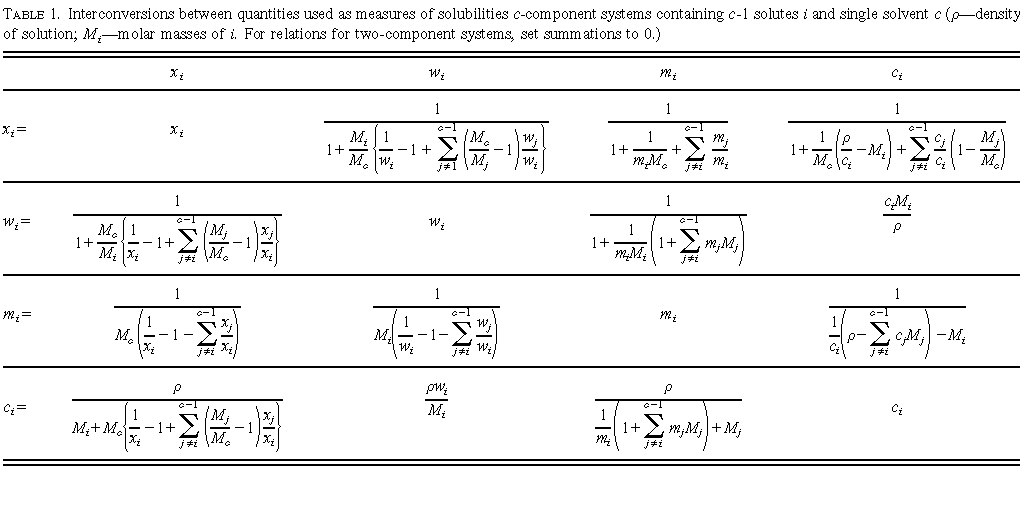
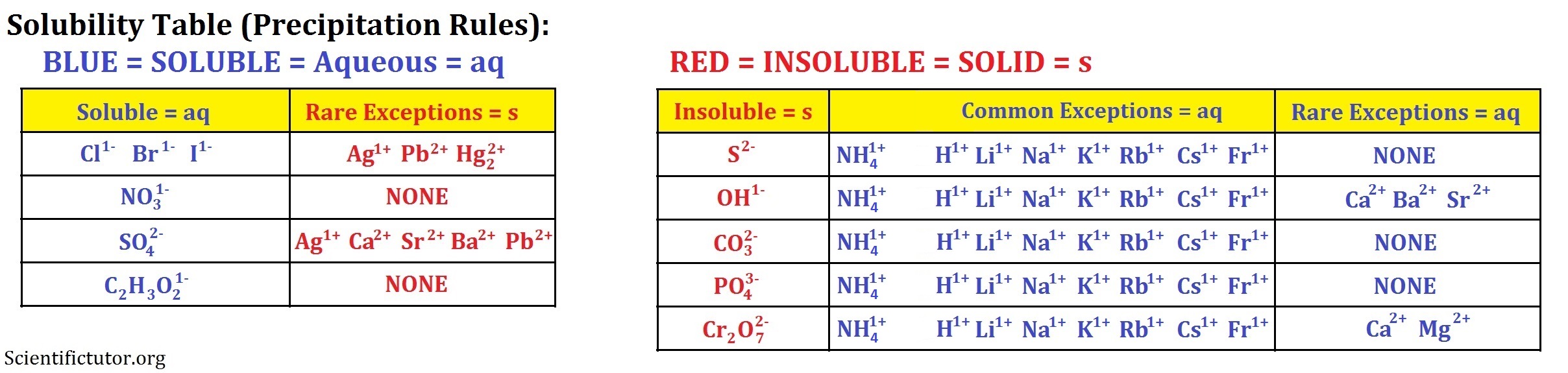
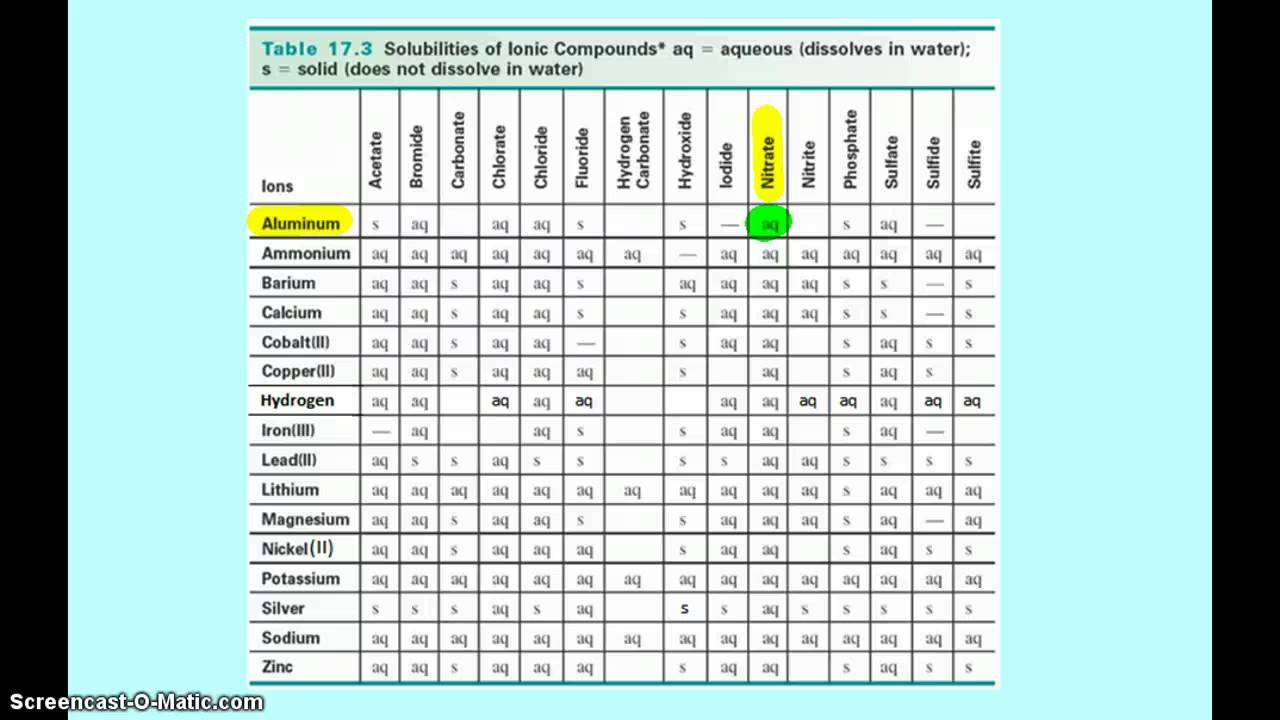
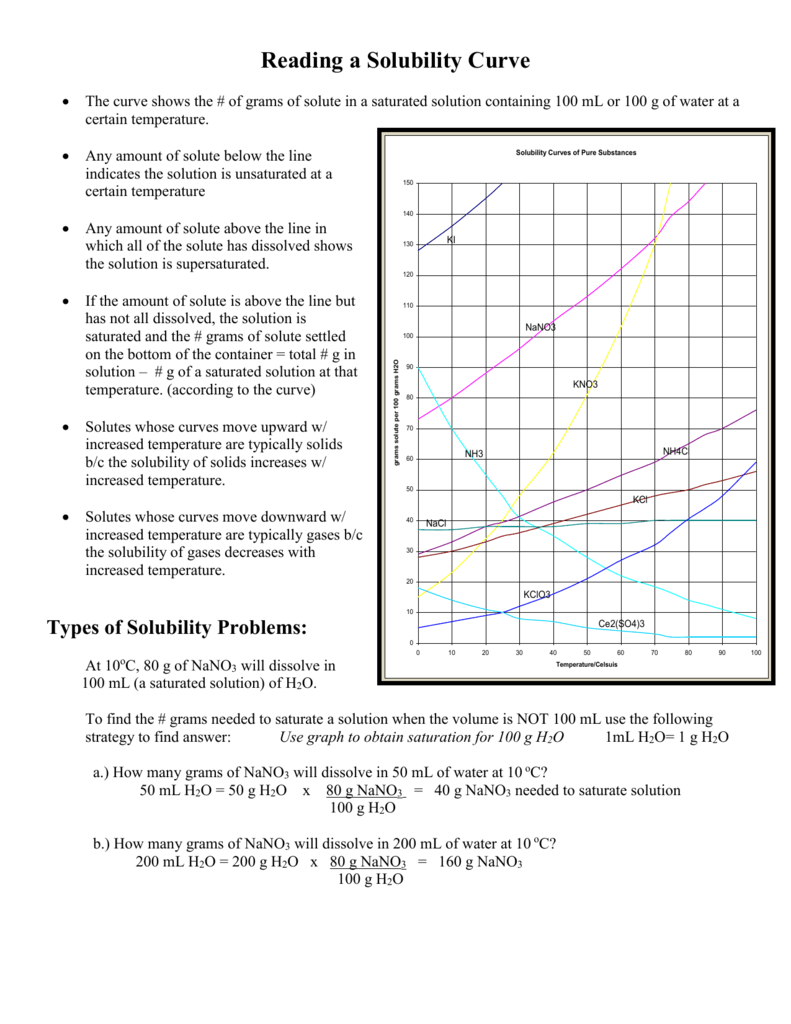
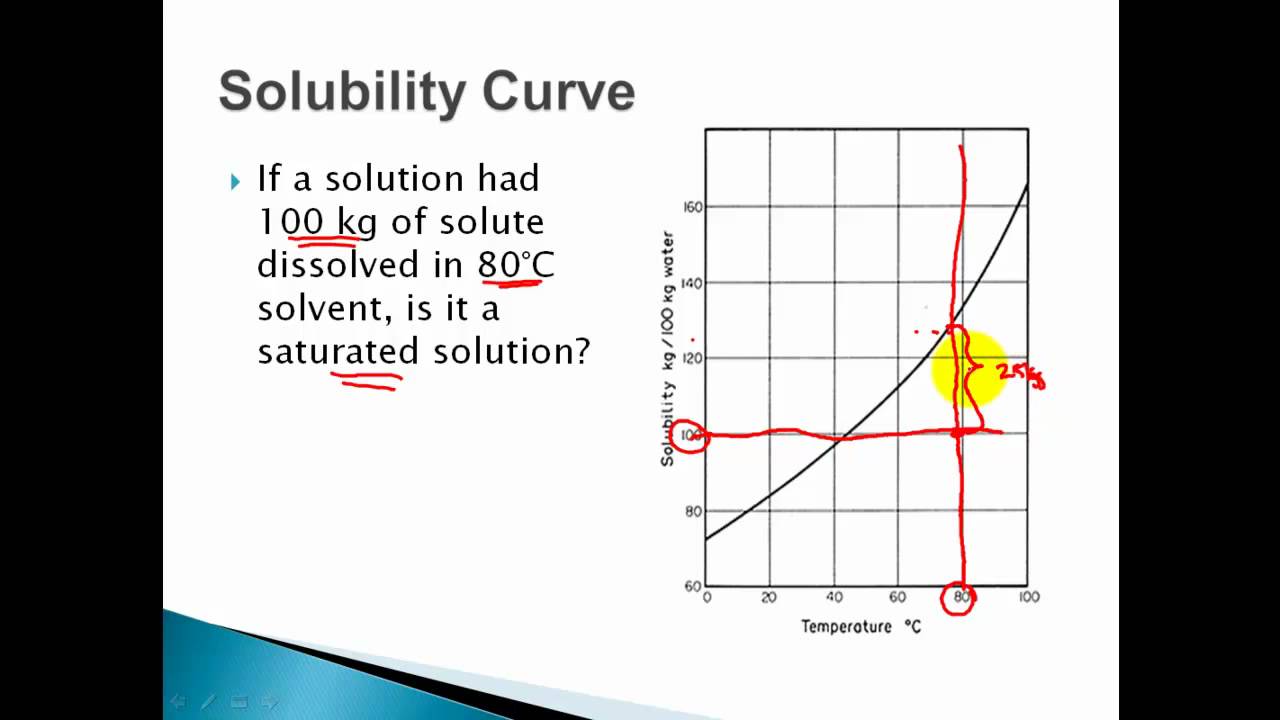
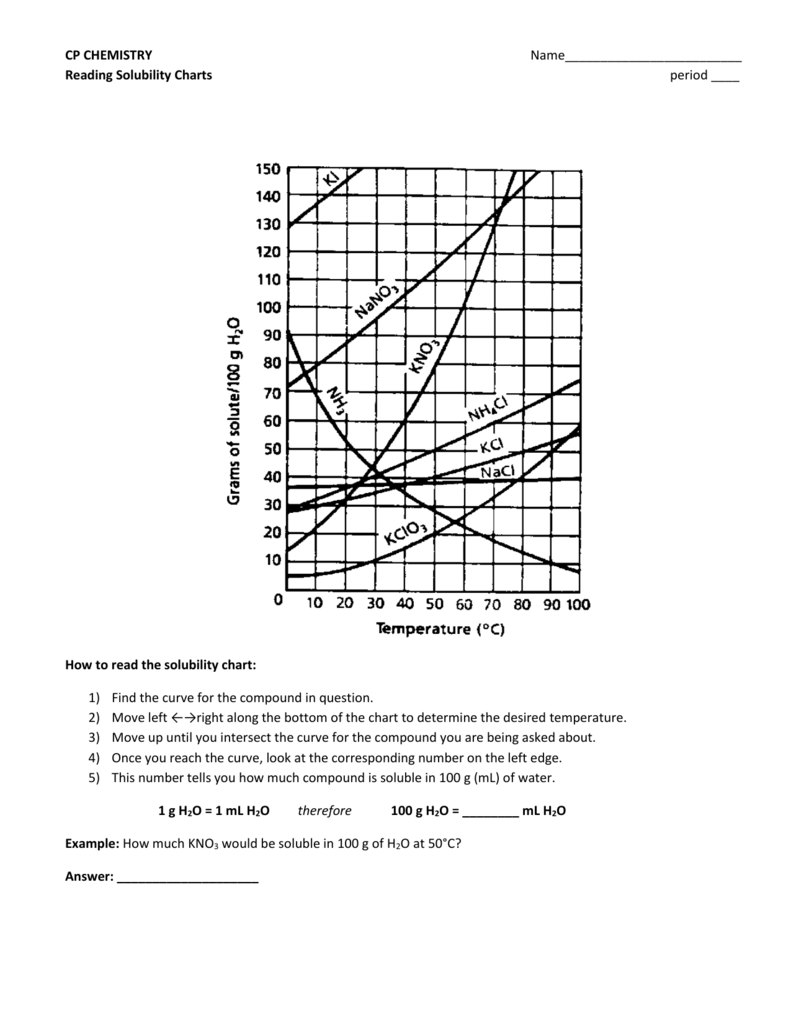
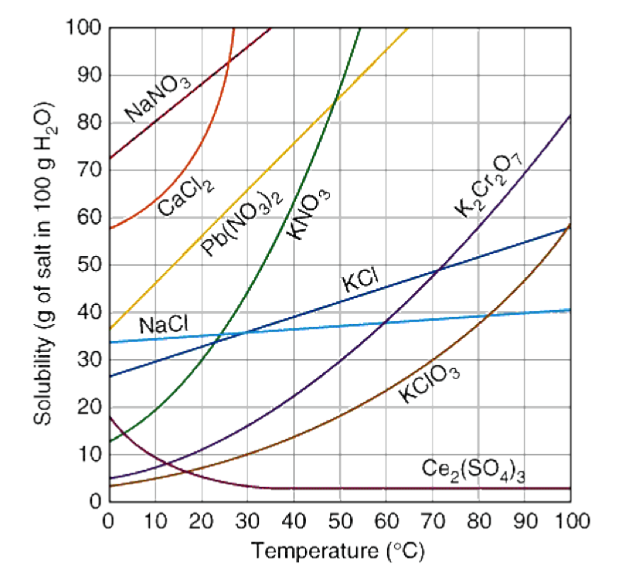
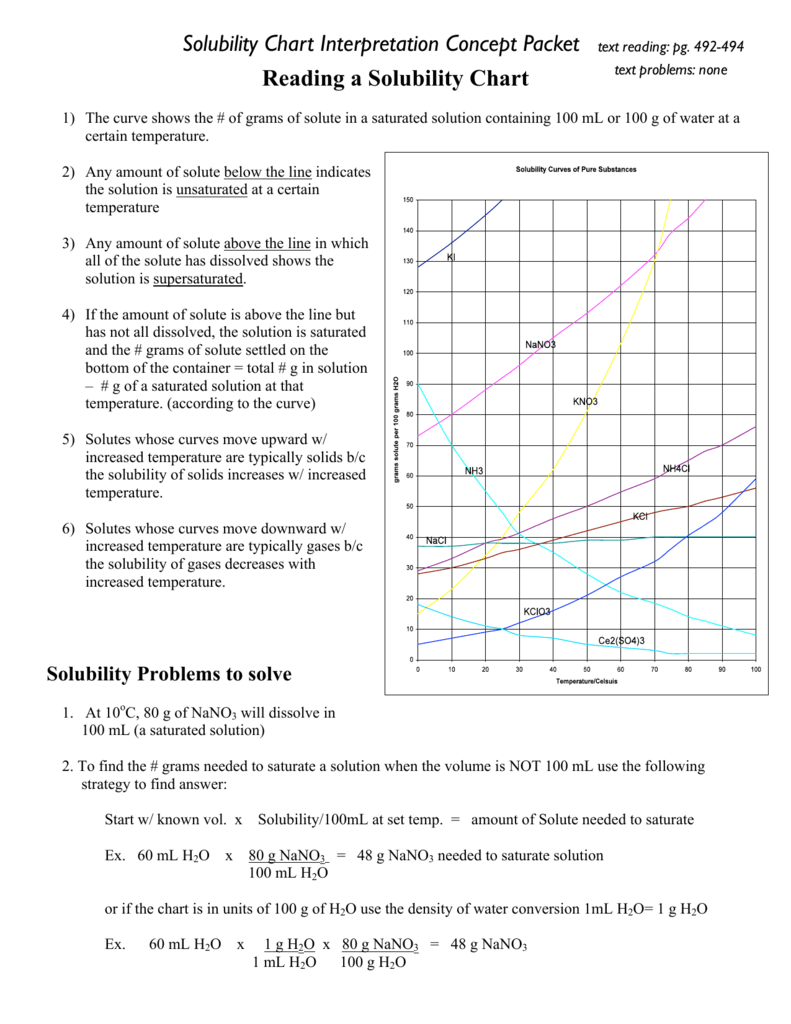
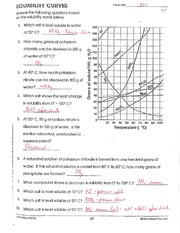
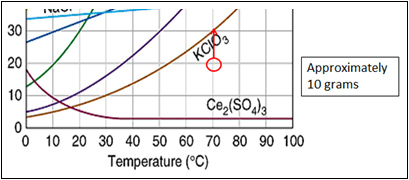
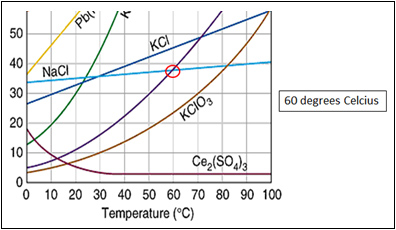
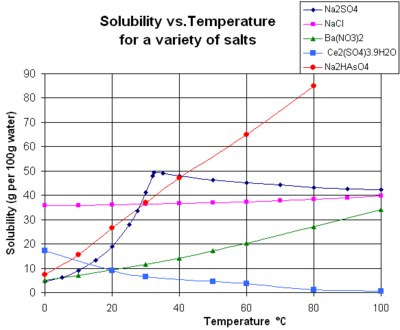
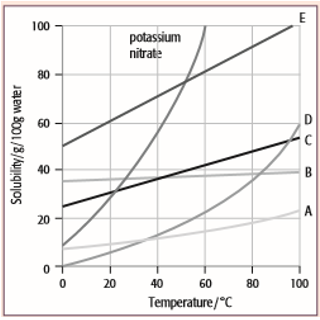
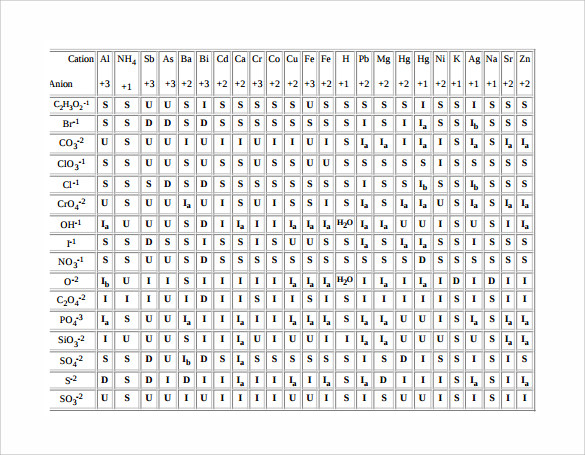
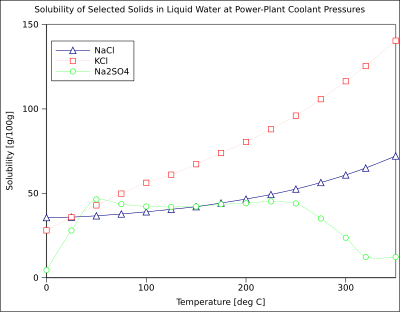
How to read a solubility chart. Use graph to determine mass of solute that dissolves in. How to use the solubility table the solubility table on pages 61 62 in the chemistry 0861 lab manual can be used to predict whether or not a given ionic compound is soluble. 1 the curve shows the of grams of solute in a saturated solution containing 100 ml or 100 g of water at a certain temperature.
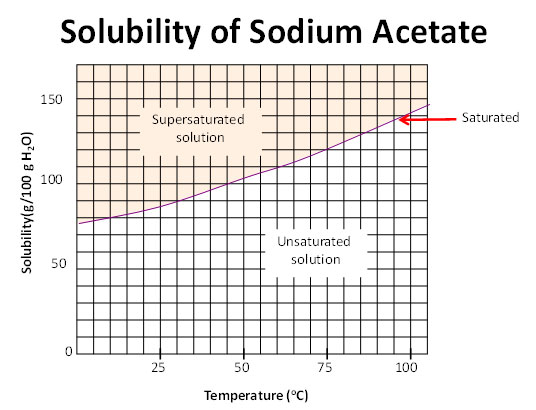
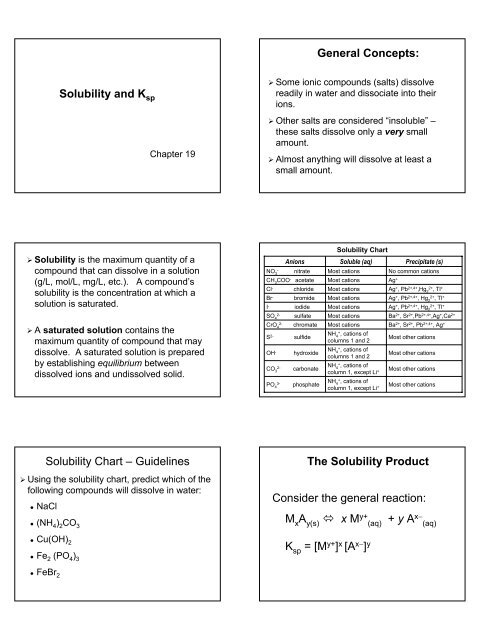
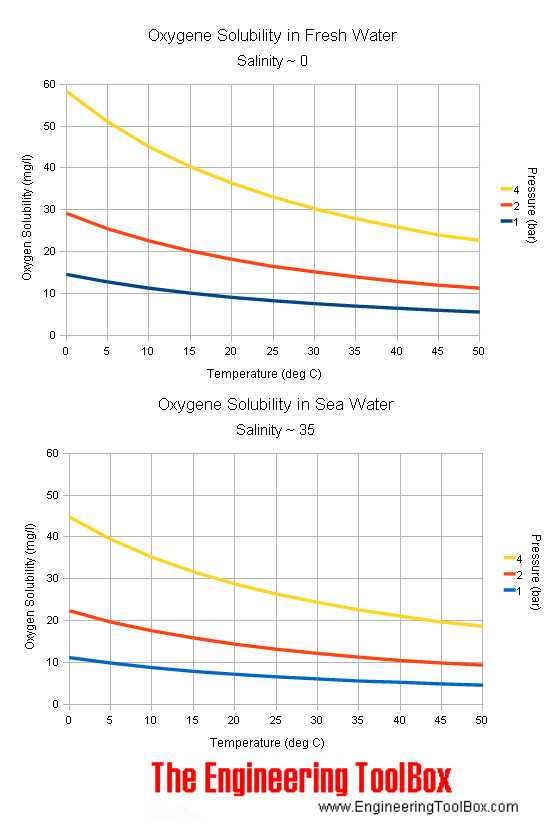
Identification of saturated unsaturated and supersaturated are included p. There are several solutes both salts and gasses identified in the table. Reading a solubility chart the curve shows the of grams of solute in a saturated solution containing 100 ml or 100 g of water at a certain temperature.
This lesson introduces a solubility chart comparing temperature and solubility of several compounds with explanation of how to read the chart. In this video i go over all of the solubility rules plus we do a bunch of examples predicting the. 2 any amount of solute below the line indicates the solution is.
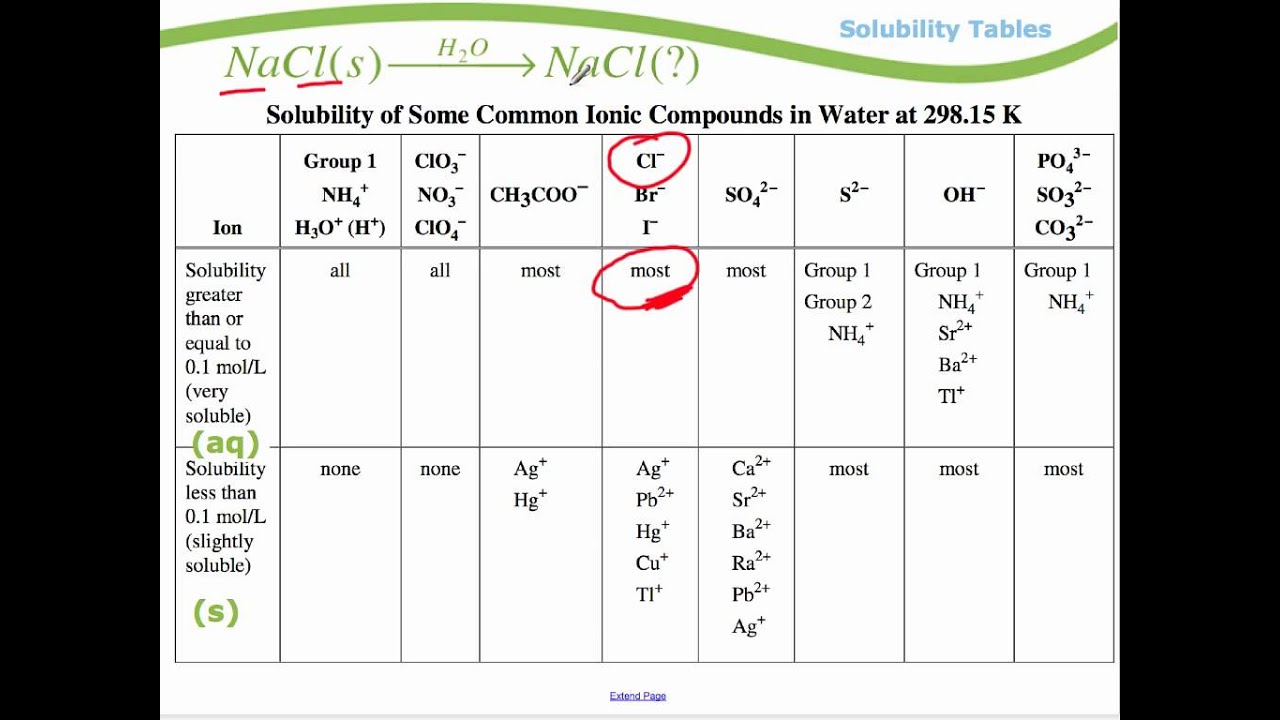
So solubility can be difficult if you don t know how to properly use a solubility table. General procedure 1 identify the anion the negative ion in your compound. The best answer i can provide for you is this video demonstration.
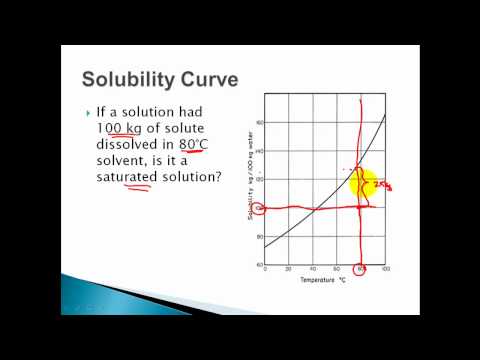
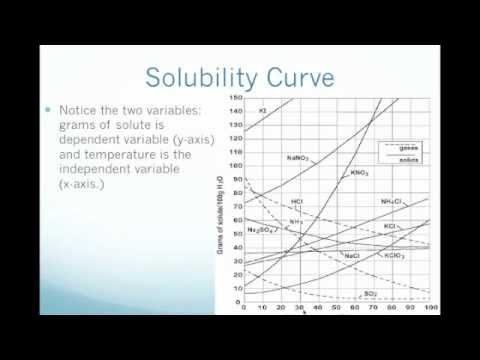
2 find the row of the table that mentions the anion in the first column. Quoting now from an australian website the ratio of mass of solute to mass of water remains the same at a given temperature. The video explains how to read a solubility graph based upon temperature in 100 grams of water.
We explain solubility chart with video tutorials and quizzes using our many ways tm approach from multiple teachers. 3 any amount of solute above the line in which all of the solute has dissolved shows the solution is. The typical solubility curve shows the grams of a solute that will saturate 100 gram of water.
Any amount of solute below the line. I hope this was helpful.














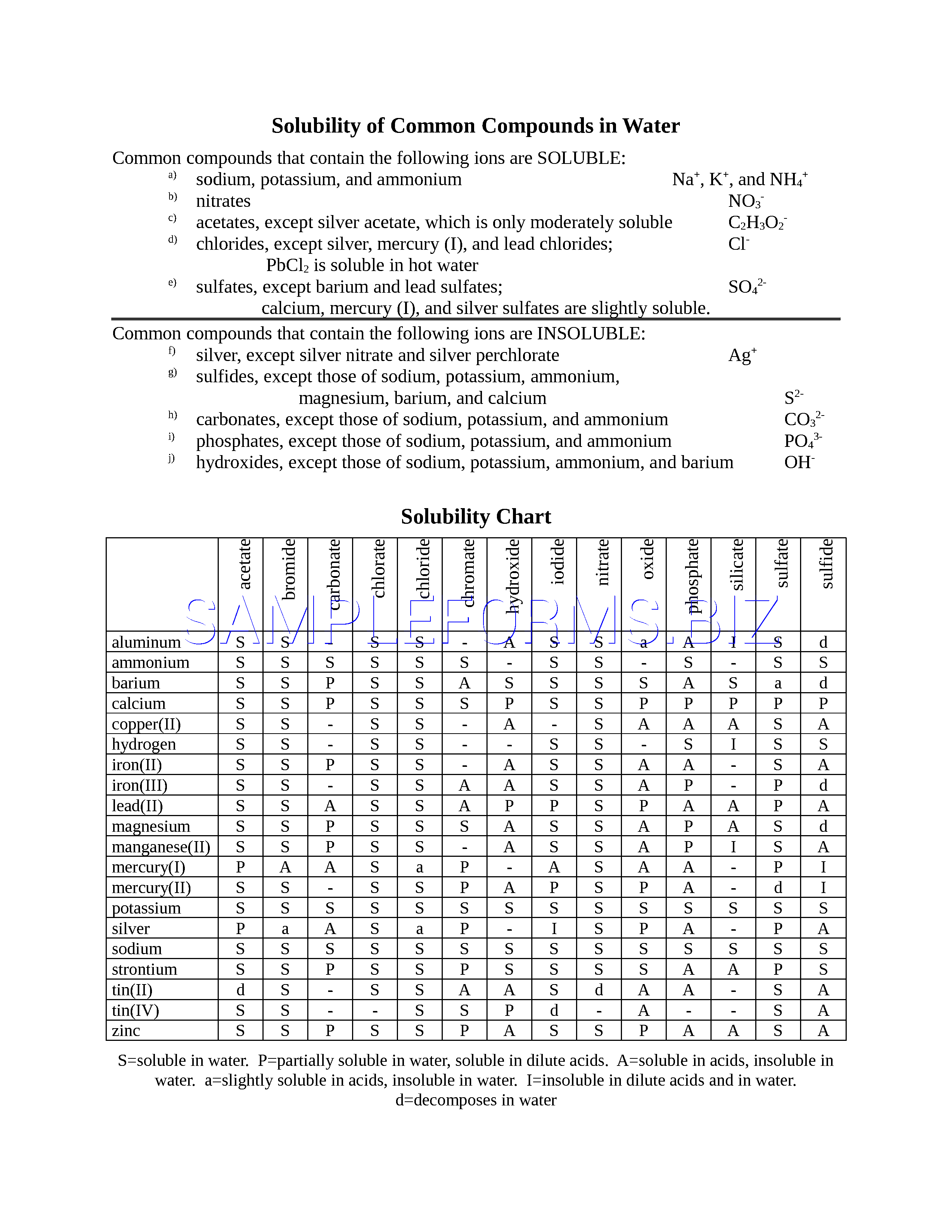


.png)